The Hunger Games: How Ghrelin and Leptin Control Your Appetite and Weight
The Hidden Chemistry That Drives Your Cravings and Satisfaction
Welcome back, nutrition enthusiasts! If you've been following my weight loss series, you've already learned about the glycemic index and how low vs. high GI foods affect your body differently. We've explored the science of intermittent fasting, debated zero-calorie sweeteners, and dug deep into the roles of fiber, protein, and carbohydrates in your diet. But today, we're going to peek behind the curtain at the hormonal puppeteers that secretly pull the strings of your hunger and satiety: ghrelin and leptin.
Think of these two hormones as the angel and devil on your shoulders when it comes to appetite control. One whispers "Eat! Eat! Eat!" while the other gently suggests "You've had enough." Understanding this hormonal tug-of-war might just be the missing piece in your weight management puzzle. So let's dive into the fascinating world of these hunger hormones and discover how they influence your eating behavior, weight, and overall health.
Meet Your Hunger Hormones: The Dynamic Duo
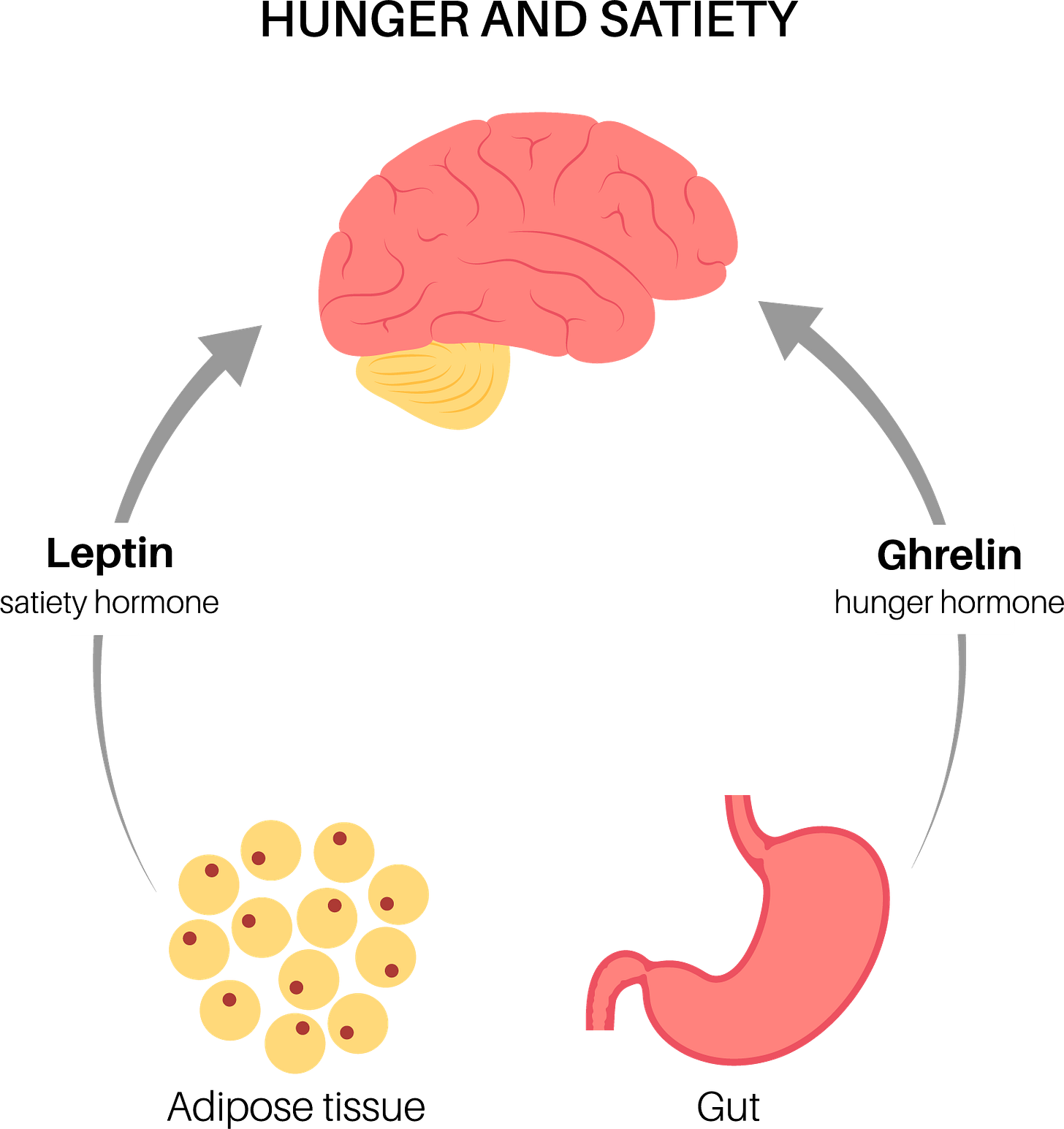
Ghrelin: Your Stomach's Hunger Signal
Often dubbed the "hunger hormone”, ghrelin is primarily produced by your stomach when it's empty (Cleveland Clinic, n.d.). Think of ghrelin as that impatient friend who keeps checking their watch and asking, "Isn't it time to eat yet?" Its main job is to increase your appetite by signaling your brain that you're hungry.
Ghrelin isn't just made in your stomach, though. Smaller amounts are also produced by your brain, small intestine, and pancreas. Besides stimulating appetite, this multitasking hormone:
Increases food intake and helps your body store fat
Triggers your pituitary gland to release growth hormones
Plays a role in regulating blood sugar and insulin release
Supports muscle strength and bone formation
Stimulates your digestive system to move food through your intestines
Once produced, ghrelin needs to connect with special receivers in your body called receptors. Think of these as docking stations where ghrelin can land and deliver its "I'm hungry" message. These receptors are found throughout your body but are especially important in your brain's appetite center.
Your ghrelin levels aren't constant throughout the day. They typically peak right before mealtimes (signaling hunger) and decrease after you've eaten (telling your brain you're satisfied). This preprandial increase in ghrelin correlates strongly with self-reported hunger scores in humans, often initiating meals voluntarily even in the absence of time- and food-related cues (Klok et al., 2007).
Leptin: The Satiety Sentinel
If ghrelin is the "eat more" hormone, leptin is its counterbalance—the "you're full" hormone. Discovered in 1994, leptin is primarily produced by your adipose tissue (body fat) and signals to your brain about your long-term energy stores (Zhang et al., 1994).
Unlike ghrelin, which fluctuates throughout the day based on your meal timing, leptin provides a broader picture of your energy balance. Its main functions include:
Suppressing food intake
Increasing energy expenditure
Regulating the long-term balance between food intake and energy use
Influencing your metabolism, endocrine system, and immune system
As leptin is produced by fat cells, the amount in your bloodstream directly correlates with your body fat percentage. More body fat means more leptin in your blood, which should theoretically signal your brain (hypothalamus) that you have sufficient energy stores and don't need to eat more. But as we'll see later, this system doesn't always work as designed, especially in people with obesity.
How Do These Hormones Communicate With Your Brain?
Ghrelin's Path to Your Brain
When your stomach releases ghrelin, it travels to your brain through multiple routes:
Through your bloodstream: Ghrelin enters your bloodstream and travels to your brain, crossing what's called the blood-brain barrier (a protective layer that shields your brain).
Through nerve connections: Ghrelin can send signals through the vagus nerve—a direct communication line between your digestive system and brain.
Local production: Some ghrelin is actually made directly in your brain, allowing for immediate local signaling (Klok et al., 2007).
When ghrelin reaches your brain's appetite center, it basically flips the "hunger switch" to ON. It activates the brain cells that make you feel hungry while turning down the activity of cells that would normally signal fullness. The result? You start thinking about food and feel motivated to eat.
Leptin's Journey
Leptin works like ghrelin's opposite:
Your fat cells release leptin into your bloodstream.
Leptin travels to your brain and crosses into the appetite control center.
There, leptin binds to its receptors and delivers the message: "We have enough energy stored, so you can stop eating now."
Leptin essentially turns down the "hunger switch" that ghrelin turns on. It decreases activity in brain cells that stimulate appetite while activating brain cells that signal fullness (Klok et al., 2007).
Think of these hormones as a tag team working opposite shifts—ghrelin is on duty when your stomach is empty, while leptin takes over when your energy stores are sufficient.
The Regulatory Controls: What Affects These Hormone Levels?
Ghrelin's Regulators
Several factors influence how much ghrelin your body produces:
Food intake: Levels decrease after eating
Age: Decreases with increasing age
Gender: Higher in females compared to males
BMI: Decreases with increasing BMI
Growth hormone: Decreases ghrelin
Glucose: Decreases ghrelin
Insulin: Decreases ghrelin
Leptin's Influencers
Similarly, leptin production responds to various factors:
Energy stores: Increases with higher BMI and body fat percentage
Food intake: Increases with feeding
Gender: Higher in females compared to males
Age: Decreases with increasing age
Exercise: Decreases leptin
Glucose uptake: Increases leptin
Sleep: Leptin follows a diurnal pattern, with levels typically higher during sleep
How These Hormones Control Your Weight
Ghrelin
When your ghrelin levels rise, you want to eat. If these levels stay high all the time, you might find yourself constantly snacking, which can lead to weight gain over time.
Here's where things get interesting: People with obesity actually have lower levels of ghrelin than thin people (Cummings et al., 2002). That's the opposite of what you might expect! Scientists believe this happens because the body is trying to adapt—when you already have plenty of energy stored as fat, your body produces less of the "I'm hungry" signal.
But there's a catch. When people with obesity lose weight, their ghrelin levels increase, almost as if their body is trying to get that weight back. This is one reason maintaining weight loss can be so challenging—your hunger hormone is working against you.
Leptin
When leptin was discovered in 1994, scientists got very excited. They thought they'd found the solution to obesity! The theory was simple: Leptin tells your brain you're full, so giving people more leptin should reduce appetite and promote weight loss.
For people with a rare condition called congenital leptin deficiency (where the body can't produce leptin), this works perfectly. When these individuals receive leptin treatment, their appetite decreases, they lose weight, become more active, and their metabolism improves (Montague et al., 1997).
But for most people with obesity, there's a frustrating twist—they already have high levels of leptin in their blood (because they have more fat cells producing it), yet they still feel hungry. This suggests something is blocking leptin's "I'm full" message from getting through.
The Leptin Resistance Problem
This brings us to one of the most puzzling aspects of obesity—leptin resistance. Think of it like this: If leptin is a fire alarm telling you to stop eating, in people with obesity, the alarm is ringing loudly, but the brain has essentially put in earplugs.
Here's what might be happening:
The message can't get through: In some cases, leptin can't cross from the bloodstream into the brain efficiently in people with obesity.
The receiver is broken: Sometimes leptin reaches the brain, but the receiving cells have stopped responding properly to the signal.
Inflammation interferes: The low-grade inflammation that often accompanies obesity can disrupt leptin's signaling.
Most importantly, this resistance seems to develop after weight gain begins, not before. It's more a consequence of obesity than its initial cause, but once established, it makes weight loss much harder.
Intermittent Fasting and Your Hunger Hormones
As I detailed in my previous blog post on intermittent fasting, this eating pattern can lead to numerous health benefits. Now, let's connect those benefits specifically to ghrelin and leptin—the hormonal players behind the scenes.
When you first start fasting, ghrelin levels spike at your usual mealtimes, making you hungry. But here's the fascinating part: your body adapts over time. Those ghrelin spikes become less pronounced as your body resets its hunger signals to your new eating pattern (Klok et al., 2007).
Meanwhile, leptin levels drop significantly during fasting—an ancient survival signal telling your body to conserve energy. Despite this apparent contradiction for weight loss, intermittent fasting still helps metabolically by improving insulin sensitivity, reducing inflammation, enhancing fat-burning capabilities, and triggering cellular cleanup processes. These benefits help explain why fasting can improve metabolic health beyond just calorie reduction (de Cabo & Mattson, 2019).
For a deeper dive into intermittent fasting protocols and benefits, check out my comprehensive guide in my previous blog post.
Glycemic Index and Hunger Signals
As regular readers know from my glycemic index blog post, the GI of foods affects your blood sugar response—but it also directly impacts your hunger hormones.
High-GI foods create a hormonal rollercoaster: They temporarily suppress ghrelin with a quick insulin surge, but the subsequent blood sugar crash brings ghrelin roaring back prematurely, triggering hunger sooner. In contrast, low-GI foods create steady, gradual changes in ghrelin, promoting longer-lasting fullness.
Research in the American Journal of Clinical Nutrition confirms this relationship: high-GI meals caused bigger initial drops in ghrelin but led to higher ghrelin levels (and more hunger) later compared to low-GI meals (Apolzan & Harris, 2011).
This hormonal connection adds another powerful reason to choose low-GI foods, as explained in detail in my previous glycemic index post.
Gastric Bypass Surgery and Ghrelin: A Dramatic Change
For people with severe obesity who haven't succeeded with diet and lifestyle changes alone, bariatric surgery is sometimes recommended. These surgeries create major changes in hunger hormone levels.
How Surgery Affects Your Hunger Hormones
After gastric bypass surgery or sleeve gastrectomy (two common weight-loss surgeries), patients typically experience much lower ghrelin levels (Cleveland Clinic, n.d.). This helps explain why many patients report dramatically reduced hunger after surgery.
This happens for several reasons:
Smaller stomach: Since the stomach produces most of your ghrelin, removing a portion of it directly reduces ghrelin production.
Changed digestion patterns: The surgery changes how quickly food moves through your digestive system, affecting various hunger signals.
Hormone adjustments: The surgery impacts several digestive hormones beyond just ghrelin.
These hormonal changes help explain why bariatric surgery is often more successful for long-term weight loss than dieting alone. It's not just about having a smaller stomach—it's about fundamentally changing your hunger signals.
Health Conditions Related to Ghrelin and Leptin
Various health conditions can affect your hunger hormone levels, sometimes in surprising ways.
When Ghrelin Levels Go Wrong
Conditions with low ghrelin:
Obesity (surprisingly)
Certain digestive disorders like IBS and gastritis
H. pylori infection (a common stomach bacteria)
Conditions with high ghrelin:
Anorexia nervosa
Muscle wasting conditions
Celiac disease
Inflammatory bowel disease
Prader-Willi syndrome (a genetic condition that causes extreme hunger)
When Leptin Levels Are Abnormal
Conditions with high leptin (often with leptin resistance):
Obesity
Depression
Food addiction
Certain brain disorders
Fatty liver disease
Conditions with low leptin:
Rare genetic leptin deficiency
Abnormal fat distribution conditions
Certain types of amenorrhea (missed periods)
Anorexia nervosa
The Sleep Connection: Rest and Your Hunger Hormones
If you've ever noticed you're hungrier after a poor night's sleep, your hunger hormones are to blame.
Research has found that sleep-deprived people have about 15% higher ghrelin levels and 15% lower leptin levels compared to well-rested people (Spiegel et al., 2004). This hormonal shift increases hunger, particularly for high-calorie, carb-heavy foods (hello, donut cravings!).
This happens for several reasons:
Both leptin and ghrelin follow daily rhythm patterns that get disrupted when you don't sleep enough.
Sleep deprivation increases stress hormones like cortisol, which can affect hunger hormone production.
Poor sleep interferes with how your body processes sugar, indirectly affecting hunger hormone regulation.
As our society has trended toward shorter sleep duration, this hormonal disruption may be contributing to increased food intake and weight gain on a population level.
The Key Takeaways
Understanding the complex dance between ghrelin and leptin offers valuable insights for anyone struggling with weight management. These hormones don't operate in isolation—they're part of an intricate network influenced by diet, physical activity, sleep, stress, and even your gut microbiome.
The key takeaways from our exploration of hunger hormones include:
Ghrelin and leptin work in opposition to regulate appetite and energy balance, with ghrelin stimulating hunger and leptin promoting satiety.
Obesity is characterized by leptin resistance, where the brain no longer responds properly to leptin's satiety signals despite high circulating levels.
Diet composition matters for hormone balance, with protein and low-GI carbohydrates generally providing better ghrelin suppression.
Intermittent fasting may help reset hunger hormone patterns and improve metabolic health beyond simple calorie restriction.
Sleep quality and quantity significantly impact hunger hormone balance, with poor sleep shifting the balance toward increased hunger.
Bariatric surgery produces sustained weight loss partly through its effects on reducing ghrelin production.
But this is just the beginning of your journey to hormonal harmony. In my next blog post, I'll share practical, evidence-based strategies to optimize your ghrelin and leptin levels naturally. You'll discover exactly which foods can help suppress ghrelin and boost leptin sensitivity, how to time your meals for optimal hormone response, and specific lifestyle changes that can help break the cycle of leptin resistance.
Want to stop fighting your body's hormones and start working with them instead? Subscribe to my blog to make sure you don't miss these game-changing insights that could transform your relationship with food and finally make weight management feel less like a constant battle. Your hunger hormones may be powerful, but with the right knowledge, you can take back control!
References
Apolzan, J. W., & Harris, R. B. (2011). Differential effects of chow and purified diet on the consumption of sucrose solution and lard and the development of obesity. Physiology & Behavior, 105(2), 325-331.
Banks, W. A., Kastin, A. J., Huang, W., Jaspan, J. B., & Maness, L. M. (1999). Leptin enters the brain by a saturable system independent of insulin. Peptides, 17(2), 305-311.
Cleveland Clinic. (n.d.). Ghrelin: What it is, function & what it does. Retrieved from https://my.clevelandclinic.org/health/articles/22138-ghrelin
Cummings, D. E., Weigle, D. S., Frayo, R. S., Breen, P. A., Ma, M. K., Dellinger, E. P., & Purnell, J. Q. (2002). Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. New England Journal of Medicine, 346(21), 1623-1630.
de Cabo, R., & Mattson, M. P. (2019). Effects of intermittent fasting on health, aging, and disease. New England Journal of Medicine, 381(26), 2541-2551.
Howard, J. K., Cave, B. J., Oksanen, L. J., Tzameli, I., Bjørbæk, C., & Flier, J. S. (2004). Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nature Medicine, 10(7), 734-738.
Klok, M. D., Jakobsdottir, S., & Drent, M. L. (2007). The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obesity Reviews, 8(1), 21-34.
Kolaczynski, J. W., Considine, R. V., Ohannesian, J., Marco, C., Opentanova, I., Nyce, M. R., ... & Caro, J. F. (1996). Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes, 45(11), 1511-1515.
Levin, B. E., Dunn-Meynell, A. A., & Banks, W. A. (2004). Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 286(1), R143-R150.
Lo, K. M., Zhang, J., Sun, Y., Morelli, B., Lan, Y., Lauder, S., ... & Das, B. (2005). Engineering a pharmacologically superior form of leptin for the treatment of obesity. Protein Engineering, Design and Selection, 18(1), 1-10.
Montague, C. T., Farooqi, I. S., Whitehead, J. P., Soos, M. A., Rau, H., Wareham, N. J., ... & O'Rahilly, S. (1997). Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature, 387(6636), 903-908.
Spiegel, K., Tasali, E., Penev, P., & Van Cauter, E. (2004). Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine, 141(11), 846-850.
Tschöp, M., Smiley, D. L., & Heiman, M. L. (2000). Ghrelin induces adiposity in rodents. Nature, 407(6806), 908-913.
Weigle, D. S., Cummings, D. E., Newby, P. D., Breen, P. A., Frayo, R. S., Matthys, C. C., ... & Purnell, J. Q. (2003). Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. The Journal of Clinical Endocrinology & Metabolism, 88(4), 1577-1586.
Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., & Friedman, J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature, 372(6505), 425-432.